SUMMARY: Having missed a possible harm signal for the Oxford AstraZeneca COVID-19 vaccine, the delays in taking action continue
After the great interest shown by the UK Parliament, our readers, and the MHRA's enablers, we continue reviewing the minutes of the 26 April 2021 meeting of the MHRA’s Commission On Human Medicines (CHM) COVID-19 Vaccines Benefit Risk Expert Working Group. Oops…. the Minutes have been withdrawn in the interests of transparency, silly us. Never mind; here is the PDF.
We have skipped the 23 April meeting as its content adds nothing to the theme of delay.
Its one memorable sentence is, “The number of women who have received the vaccine whilst breastfeeding is not currently known”. Considering that such a population was excluded from the trials, we find the complete ignorance of coverage and effects quite striking.
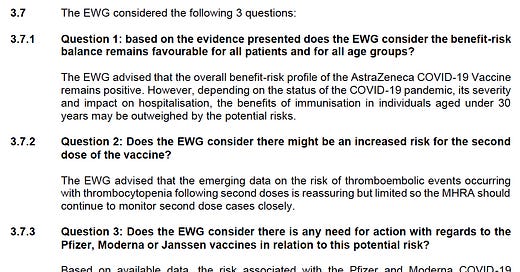
Anyway, back to the 26 April 2021 meeting. During this meeting, the secret squirrels discussed (well, we are not sure they discussed anything, but let’s…
Keep reading with a 7-day free trial
Subscribe to Trust the Evidence to keep reading this post and get 7 days of free access to the full post archives.