The H5N1 vaccine UKHSA purchased from CSL/Sequirus LTD
Maybe
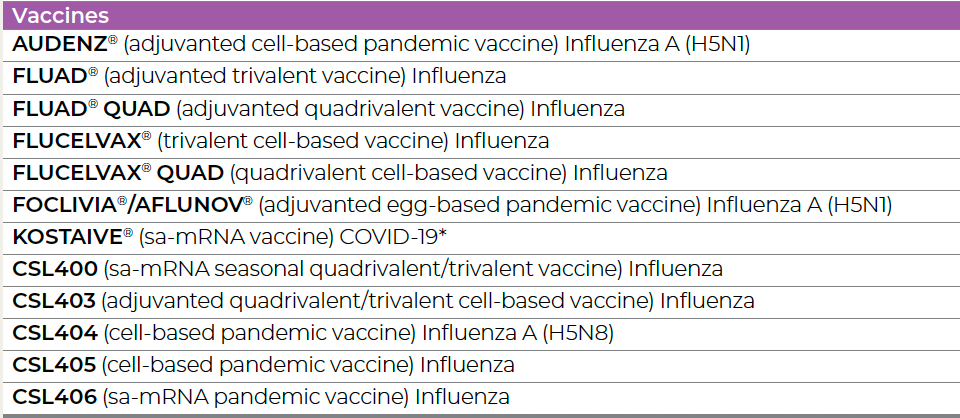
The UKHSA purchased over 5 million doses of an Influenza H5N1 pandemic vaccine from CSL/Sequirus LTD. The UKHSA is highly secretive about its actions, so we have had to work out for ourselves that the stuff they bought or optioned is most likely AUDENZ (In Europe, AUDENZ is called Incellipan):
This is taken from page 23 of CSL's Annual Report to Investors 2024. You will be interested to know that the FDA has not licensed any of the CSL4 series influenza vaccines so far.
So, let’s assume the British taxpayer has invested in Audenz. The FDA licensed the vaccine on 24 April 2024 “for use in persons 6 months of age and older at increased risk of exposure to the influenza A virus H5N1 subtype contained in the vaccine.”
Mark the words, folks.
As you know, we look at regulatory data, not commercial publications in biomedical journals, so we went to the US FDA documentation. It’s worth looking at the accelerated approval letters, which show the progressive development of the marketing plan ove…
Keep reading with a 7-day free trial
Subscribe to Trust the Evidence to keep reading this post and get 7 days of free access to the full post archives.