SUMMARY. The dataset is full of gaps, it’s unclear what the MHRA Expert Working Group based their decisions on and it’s all secret. But everything else is OK
We continue reviewing the minutes of the meetings of the MHRA’s Commission On Human Medicines (CHM) COVID-19 Vaccines Benefit Risk Expert Working Group, which took place between 20 and 27 November 2020.
Our secret squirrels were busy during this time, holding four meetings. We cannot comment on the 20 and 21 November meetings as the EWG heard presentations from sundry branches of the NHS and pharma. According to secret squirrel rules, these presentations self-destructed once delivered, and when all the industry peeps turned up on the 20th, the COI reporting went missing.
On 24 November -The EWG heard that responses to the 13 non-clinical questions posed to the company in October 2020 are awaited. (our emphasis).
It’s unclear if these 13 questions are part of the 36 questions posed by the MHRA and whether there is an obligation to respond, given the MHRA confirmed there is no formal obligation to reply in the 18th November minutes.
The EWG noted the lack of data on reproductive toxicity and histopathology and agreed the experts would review and discuss the available data with the non-clinical assessors.
The EWG discussed whether a limit should be imposed on the age of the population that can receive the vaccine, as the benefit-risk balance is less evident in younger patients.
It looks as if somebody, somewhere, was getting a little worried about the evidence gaps, even at this late stage, with emergency registration looking like a foregone conclusion. Interestingly, there is no record of the 13 non-clinical questions anywhere; could they be similar to our 15?
This reproductive age thing was exercising a few minds, as the EWG also discussed the vaccination of younger female healthcare practitioners of childbearing age and whether it would be feasible for such women to undertake a pregnancy test with the rollout of the vaccine. They noted that “at this stage it is not clear due to the lack of clinical and nonclinical data”.
This lack of data persists to this day.
Three days later, on 27 November, EWG was still discussing whether it may be necessary for women of childbearing age to undergo a pregnancy test before vaccination, as per the clinical trial population.
The EWG expressed the need to be aware of the potential cumulative effects of multiple small risks/gaps in the data (our emphasis).
However, after a presentation on the non-clinical aspects of BNT162b2 and the clinical aspects of BNT162b2, the EWG heard that the company had not provided any information on reproductive toxicity.
There is nothing to suggest that the product is teratogenic, but without data to support this, it cannot be known for certain.
The EWG also noted that the vaccine's benefits were apparently lower for the younger age groups (our emphasis).
Given the short time that the vaccine has been studied, the question of whether use in subjects under 50 years of age was justified; one member of the EWG considered that it was not, but this person’s name is not recorded, it’s all secret, you see..
The EWG discussed and concluded that the risk/benefit of COVID-19 mRNA Vaccine BNT162b2 is considered to be positive in all subjects aged 16 years and over.
Now, given that trial BNT162-01 was a phase 1 dose-ranging trial which tested BNT162b2 (which eventually became Comirnaty) with a denominator of 96 participants, we have difficulty reconciling how such a conclusion could have been drawn, especially in the absence of reproductive toxicity information, gaps in the data presented and a lack of curiosity amongst the EWG members.
Meeting minutes are a formal, legal record of a meeting that ensures transparency and accountability. In the government's guide to minute taking, the minutes should give the reasons why such conclusions were reached.
Here in the TTE Office, based on these minutes, we have no clue how these conclusions could be reached.
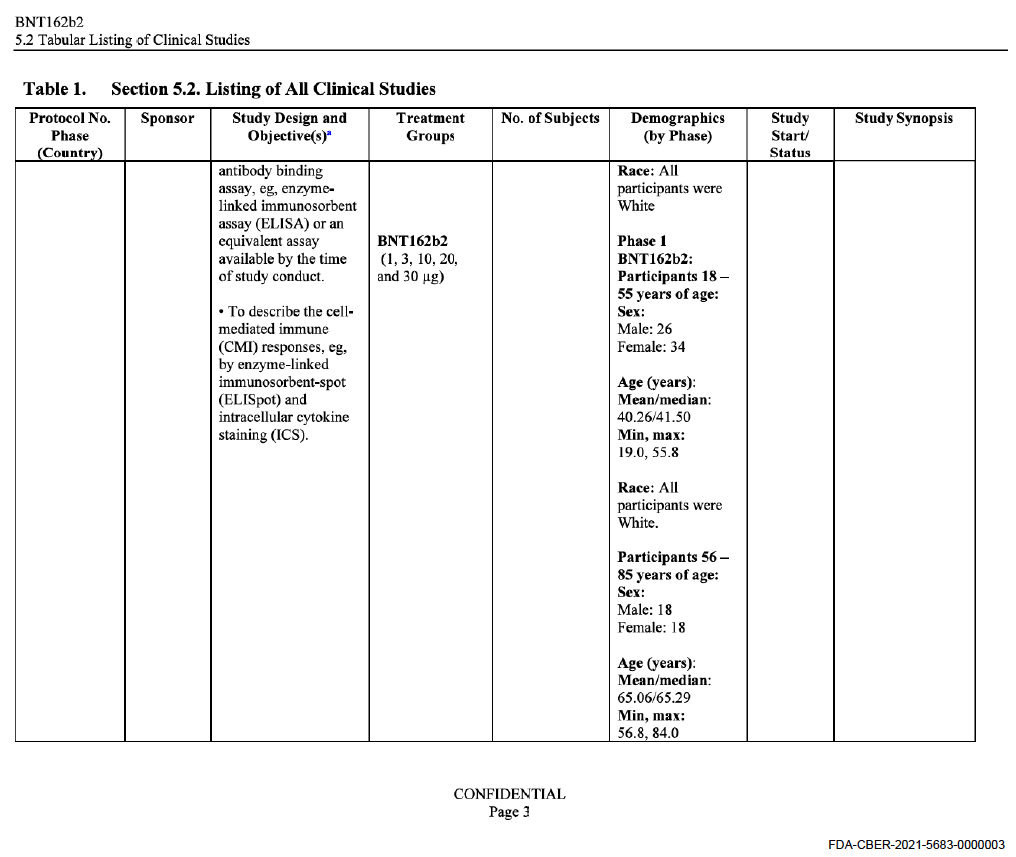
So that we are clear that the two old geezers do not tell porkies (for our US cousins, that means we do not tell lies), here is the relevant tabular view from Pfizer BionTech’s submission for Emergency Use to the US FDA:
It is possible that the summary of the minutes does not reflect a lively discussion, but readers have to bear in mind that all this was secret.
From this and the preceding posts, you get the picture of utter secrecy and lack of clarity as to what was going on during the assessment and registration of one of the most global intervention ever to receive an emergency licence.
This post was written by two old geezers who, for once, are left speechless.






So, EWG allowed to meet, but decision predetermined elsewhere? Much the same as the Ethics committee being disbanded before the decision to vaccinate 12+ years was taken by CMO meeting - JVCI wouldn't agree so passed it up to CMOs. Ethics committee against vaccinating 12+, I am told.
Is there no way we can slip the two secret squirrels through a skylight and have them bring up these questions during the Covid Inquiry? Or maybe they might look at the list of witnesses yet to be called upon and offer one or two of them a few pointers as to what was not revealed by the drug manufacturers,so that at least some of the unanswered questions might go on record???