Summary of the UKHSA and MHRA information received about the H5 Avian Influenza Vaccine.
A long read
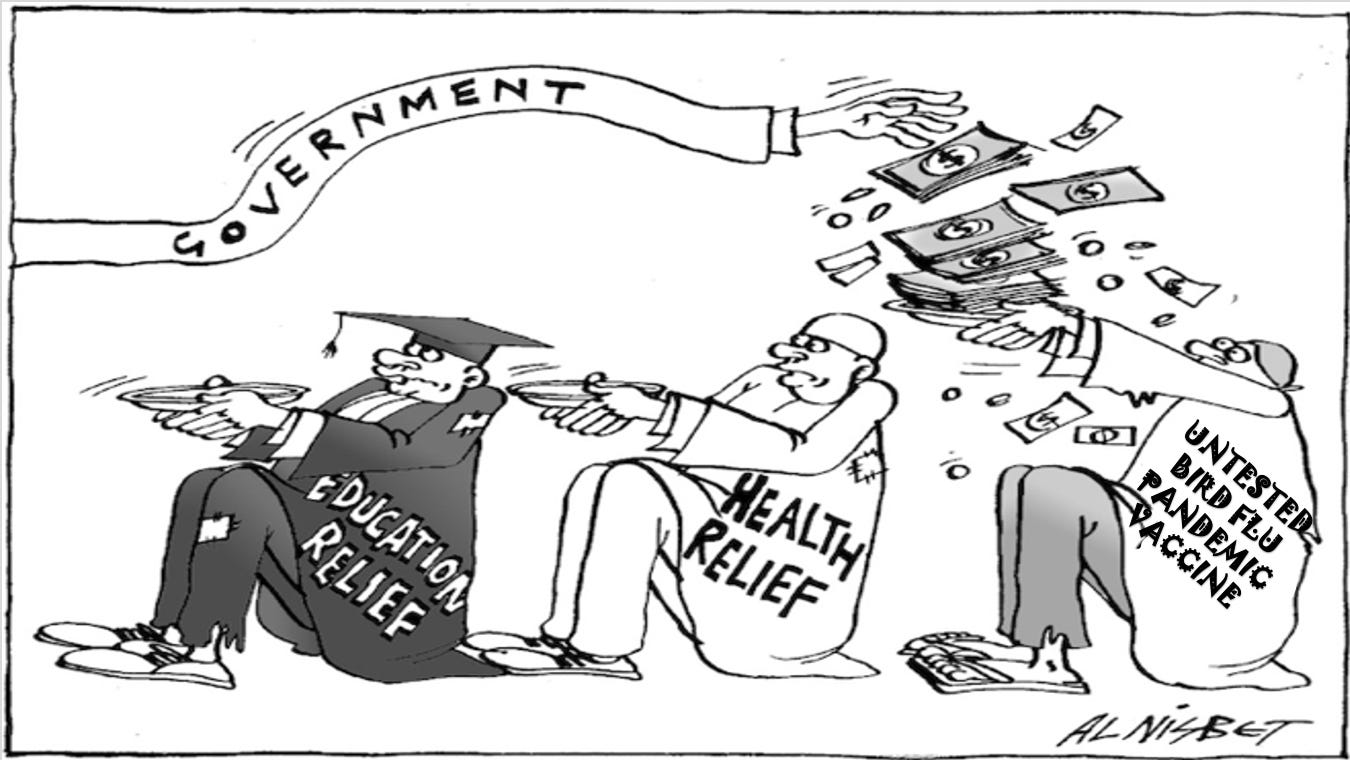
Modified from Al Nisbet
Before Christmas, we started a series of FOI requests to clarify how taxpayers' money is spent on pandemic preparedness. We were responding to the news that the UKHSA had purchased 5,147,670 doses of an Influenza H5 “pandemic” vaccine from CSL/Sequirus Ltd.
We were unconvinced by the evidence of a threat from avian influenza produc…